Abstract
Introduction: The approval of hypomethylating agents (HMAs) azacitidine (AZA) and decitabine (DAC) were major milestones in improving the clinical outcomes of patients with higher-risk myelodysplastic syndromes (MDS). In the landmark AZA-001 randomized trial, the median overall survival (OS) and the 2-year OS rates were 24.5 months and 50·8%, respectively, in the AZA arm, compared to 15 months (p=0·0001) and 26·2%, respectively, in conventional care regimen arms. However, long-term OS rates with HMAs have not been formally assessed due to limited follow-up duration of most clinical studies. To address this question, we analyzed Surveillance, Epidemiology, and End Results (SEER)-Medicare linked data to assess longer term survival for MDS patients treated with HMAs and who did not undergo transplantation.
Methods: In this retrospective cohort study, we used ICD-O-3 codes to identify MDS patients in SEER-Medicare who were diagnosed between 2004-2009 and initiated HMA before 12/31/2009. We defined patients with refractory anemia with excess blasts (RAEB) as a proxy for higher-risk MDS. Patients were followed until death or December 31, 2013, whichever came first. Our selection strategy allowed at least 4 years of potential follow-up for every patient to optimize reliability of long-term survival assessment. Patients were ≥66 years at diagnosis, had continuous Medicare Parts A and B coverage, and received ≥10 days of HMA therapy. Patients who underwent allogeneic stem cell transplantation (n=14) were excluded [Figure 1]. The Healthcare Common Procedural Coding System codes in Medicare claims identified treatments including HMAs and transfusion of red blood cells (RBC) and platelets during an 8-week period preceding the first HMA treatment. Cycles of HMA therapy were defined by at least 4 individual days of HMA use and a gap of at least 2 weeks between cycles. We measured OS from first day of HMA. Kaplan-Meier methods and log-rank test were used to analyze unadjusted survival, while the Cox proportional hazards models were used to study adjusted survival. Two-sided statistical tests with p-value <0.05 to indicate statistical significance.
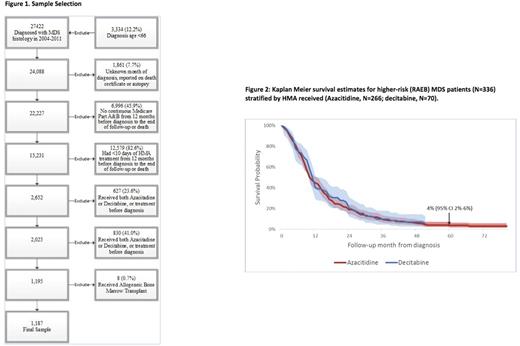
Results: We identified 1,187 eligible MDS patients [Figure 1], including 336 patients (28.3%) with RAEB.The median age at diagnosis was 77 (interquartile range [IQR] 72-81) years, and 63.8% were males. AZA was used in 936 patients (78.9%) while 251 patients (21.1%) received DAC. In total, 72.6% and 49.5% of patients received ≥4 and ≥ 6 cycles of HMA therapy, respectively, with no significant differences in these proportions between the AZA and DAC treated groups. The median OS for the entire cohort was 14 months (95% CI: 13-15 months), and the 5-year OS probability was 12% (95% CI: 10%-14%). The median OS for patients in the RAEB cohort (n=336) was 11 months from HMA initiation (95% CI: 10-12 months). The 5-year OS probability for RAEB patients was a dismal 4% (95% CI: 2%-6%). In a multivariate survival analysis, the choice of HMA was not associated with increased probability of survival among RAEB patients (DAC vs. AZA, HR = 0.96, 95% CI: 0.70-1.31, P=0.78, Figure 2) while RBC or platelet transfusions were associated with increased risk of death.
Conclusions: In this large retrospective study of 1,187 HMA-treated older patients with MDS, who had ≥ 4 years of follow-up from time of initiation of HMA therapy and who did not undergo transplantation, we observed a dismal 5-year OS probability rate of 4% (95% CI 2%-6%) for the RAEB cohort. We did not find significant difference in the probability of long-term survival as based on the type of HMA received among patients with RAEB. These results argue against the presence of a significant survival tail (or plateau) for patients higher-risk MDS after receiving HMAs. These results confirm the suboptimal performance of HMAs for treatment of MDS in the real-world setting and provide rationale for strong consideration of alternative management strategies including clinical trials for all patients with MDS.
Ma: Incyte Corp.: Consultancy. Huntington: Janssen: Consultancy; Pharmacyclics: Honoraria; Celgene: Consultancy, Other: Travel. Podoltsev: CTI biopharma/Baxalta: Consultancy; Alexion: Consultancy; Ariad: Consultancy; Incyte: Consultancy. Zeidan: Takeda: Speakers Bureau; AbbVie, Otsuka, Pfizer, Gilead, Celgene, Ariad, Incyte: Consultancy, Honoraria; Otsuka: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal